Abstract
Background: Sickle cell disease (SCD) is one of the most common genetic diseases globally. Clinical manifestations of SCD commonly include anemia, pain, vaso-occlusive events and cumulative end organ damage leading to significant morbidity and mortality. Historically, allogeneic hematopoietic cell transplantation (HCT) represented the only curative therapy for SCD, but is associated with significant HCT-related morbidity and mortality which limit its use (PMID: 8663884, PMID: 20007560).
Autologous hematopoietic stem cell (HSC) -based gene therapies now offer curative potential with decreased toxicity (PMID: 28249145). However, effective HSC-based gene therapy depends on collection of sufficient HSCs (ideally >10x106 CD34+ cells/kg), typically from peripheral blood (PB) (PMID: 33956057). G-CSF and CXCR4 inhibition (CXCR4i) with plerixafor are the most widely used mobilization strategies (PMID: 19363221, PMID: 19720922). However, G-CSF is associated with risk of fatal vaso-occlusive events in SCD (PMID: 9734950, PMID: 11368061, PMID: 19513902). Meanwhile, short-acting CXCR4i with plerixafor alone does not reliably yield optimal HSC numbers for gene therapy applications (PMID: 30282642, PMID: 29419425, PMID: 29472357). Therefore, developing novel HSC mobilization regimens to rapidly and reliably mobilize optimal CD34+ HSCs for gene therapy in SCD represents an unmet need.
Motixafortide (M) is a high-affinity, long-acting CXCR4 inhibitor. The recent Phase III, randomized, controlled GENESIS Trial demonstrated combination M+G-CSF resulted in 88.8% of patients collecting ≥6x106 CD34+ cells/kg (median 10.8x106) within 1 leukapheresis (LP) for HCT. This compares favorably to historical data with plerixafor+G-CSF where 54.2% collected to goal in 1 LP. Motixafortide also mobilized more immunophenotypically primitive HSCs and multi-potent progenitors compared to plerixafor. Meanwhile, transcriptional profiling by single-cell RNA sequencing revealed HSCs mobilized with M have gene expression profiles associated with enhanced regeneration and self-renewal, compared to G-CSF or plerixafor (DOI: 10.1182/blood-2021-144296). Motixafortide alone also mobilized an average of 11.6×106 CD34+ cells/kg in healthy volunteers, while mobilizing 91.6% of allogeneic HSC donors to goal in ≤2 LPs (median 6.07x106) (DOI: 10.1182/blood-2018-99-109701, PMID: 28835380).
Natalizumab (N) is a monoclonal antibody to the a4 integrin subunit of a4b1 (VLA-4) capable of VLA4 inhibition (VLA4i). In mice and primates, N led to an ~8-fold increase in PB CD34+ HSCs in 24 hrs (PMID: 7692447). In humans, a single N infusion resulted in a 5- to 6-fold increase PB CD34+ HSCs. Meanwhile, the average PB CD34+ HSC levels after a single dose were similar to levels with repeated doses, indicating rapid mobilization of maximal PB HSCs by 24 hrs after N infusion (PMID: 18195093).
Combination CXCR4i and VLA-4i has been well-tolerated and resulted in synergistic mobilization of HSCs in preclinical studies (PMID: 21886173). Therefore, we hypothesize that combination CXCR4i and VLA-4i with M+N represents a safe and effective strategy for robust, rapid and synergistic mobilization of HSCs to PB in patients with SCD.
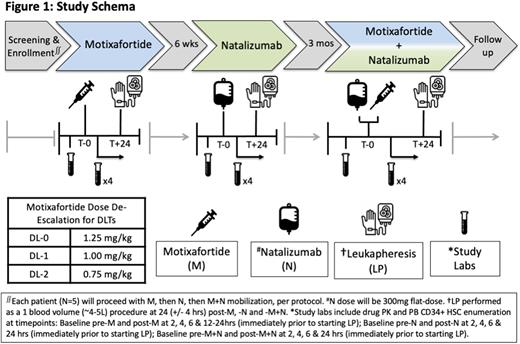
Study Design and Methods: This is a single center, single-arm, dose de-escalation, Phase 1 safety and feasibility study. Key eligibility criteria include patients with SCD (SS or Sβ0) age 18-35 years receiving automated RBC exchanges. Patients with prior HCT or gene therapy will be excluded. We estimate 5 patients in this pilot feasibility study will provide sufficient safety and preliminary efficacy data to guide further development of this novel mobilization strategy. All patients will have baseline labs prior to M (1.25mg/kg, subcutaneous), N (300mg, IV) and M+N dosing. Each patient will then undergo mobilization and LP (1 blood volume), per protocol (see Figure 1). The primary outcome will be to assess the safety and tolerability of M, N and M+N in each SCD patient, defined by dose-limiting toxicities and maximum tolerated dose. Key secondary outcomes include: kinetics of CD34+ HSC mobilization to PB (CD34+ cells/uL), number of CD34+ HSCs collected via LP (CD34+ cells/kg/L), incidence of AEs, pharmacokinetics and the recommended phase 2 dose. Correlative studies include immunophenotypic and single cell transcriptional profiling of CD34+ HSCs from LP product mobilized with M, N and M+N.
Disclosures
Crees:BioLineRx Ltd.: Research Funding. Jayasinghe:MMRF: Consultancy; WUGEN: Consultancy. Vainstein:BioLineRx Ltd.: Current Employment, Current equity holder in publicly-traded company. Sorani:BiolineRX: Current Employment. King:Global Blood Therapeutics: Consultancy, Research Funding. DiPersio:Amphivena Therapeutics: Research Funding; NeoImmune Tech: Research Funding; Macrogenics: Research Funding; BioLineRx, Ltd.: Research Funding; CAR-T cell Product with Washington University and WUGEN: Patents & Royalties; VLA-4 Inhibitor with Washington University and Magenta Therapeutics: Patents & Royalties; hC Bioscience, Inc.: Membership on an entity's Board of Directors or advisory committees; RiverVest Venture Partners: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Research Funding; WUGEN: Current equity holder in private company, Research Funding; Magenta Therapeutics: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.